Developing exclusive orthodontic products with Chinese manufacturers offers a unique opportunity to tap into a rapidly growing market and leverage world-class production capabilities. China’s orthodontics market is expanding due to increased awareness of oral health and advancements in technology like 3D imaging and AI-driven treatment planning. Additionally, the rising middle-class population and growing dental care infrastructure further fuel demand for innovative orthodontic solutions.
Manufacturers in China provide access to cutting-edge facilities and skilled labor, ensuring high-quality production at competitive costs. A strategic approach to exclusive orthodontic product development enables businesses to address market gaps effectively while protecting intellectual property and ensuring compliance with industry standards.
Key Takeaways
- Clear designs and simple drawings are important for making products. They reduce mistakes and help manufacturers know what is needed.
- Models of the product are very helpful. They show problems early and make it easier to talk with manufacturers.
- Knowing what people want is very important. Do research to find what is missing and use customer ideas in designs.
- Protect your ideas by getting patents and trademarks in your country and in China. Use agreements to keep your information private.
- Choose manufacturers wisely. Check their certificates, how much they can make, and visit their factories if possible.
Conceptualizing and Designing Exclusive Orthodontic Products
Defining Product Specifications
Importance of detailed designs and technical drawings
When developing exclusive orthodontic products, I always emphasize the importance of detailed designs and technical drawings. These serve as the foundation for translating innovative ideas into tangible products. Clear and precise designs ensure that manufacturers understand every aspect of the product, from dimensions to functionality. This level of detail minimizes errors during production and helps maintain consistency across batches.
Research supports this approach. For instance:
- Qualitative research highlights the importance of understanding customer needs and preferences, which directly influence product design.
- Effective designs can position products uniquely in the market, creating a competitive edge.
By focusing on detailed technical drawings, I ensure that the final product aligns with both market expectations and manufacturing capabilities.
Using prototypes to refine product concepts
Prototypes play a crucial role in exclusive orthodontic product development. They allow me to test and refine concepts before full-scale production. A prototype provides a physical representation of the design, enabling me to identify potential flaws and make necessary adjustments. This iterative process ensures that the final product meets the highest standards of quality and functionality.
For example, when working with Chinese manufacturers, I often use prototypes to bridge communication gaps. A tangible model helps clarify design intentions and ensures that the manufacturer fully understands the product’s requirements. This step is invaluable in achieving precision and avoiding costly revisions later.
Researching Market Needs
Identifying gaps in the orthodontic product market
Understanding market needs is essential for exclusive orthodontic product development. I start by identifying gaps in the current offerings. This involves analyzing both primary and secondary research data. For instance:
| Perspective | Primary Research | Secondary Research |
|---|---|---|
| Supplier side | Fabricators, technology purveyors | Competitor reports, government publications, independent investigations |
| Demand side | End-user and consumer surveys | Case studies, reference customers |
This dual approach helps me uncover unmet needs and emerging trends. For example, rising awareness of oral health and advancements in orthodontic technology highlight opportunities for innovative solutions.
Incorporating customer feedback into designs
Customer feedback is a cornerstone of my design process. By engaging directly with end-users, I gain valuable insights into their preferences and pain points. Surveys, interviews, and focus groups reveal what customers truly value in orthodontic products. I use this information to refine designs and ensure that the final product addresses real-world needs.
For instance, feedback from orthodontists often highlights the importance of ease of use and patient comfort. Incorporating these elements into the design not only enhances the product’s appeal but also strengthens its market position. This customer-centric approach ensures that my products stand out in a competitive landscape.
Protecting Intellectual Property in Product Development
Securing Patents and Trademarks
Steps to register intellectual property in your home country
Securing intellectual property rights is a critical step in exclusive orthodontic product development. I always begin by registering patents and trademarks in my home country to establish legal ownership. The process typically involves filing an application with the relevant intellectual property office, such as the USPTO in the United States. This application must include detailed descriptions, claims, and drawings of the product. Once approved, the patent or trademark provides legal protection, preventing unauthorized use or replication.
A robust patent strategy has proven effective for companies like Align Technology. Their patented process for digitally planning and manufacturing clear braces has been instrumental in maintaining market leadership. This example underscores the importance of securing intellectual property to sustain a competitive edge.
Understanding intellectual property laws in China
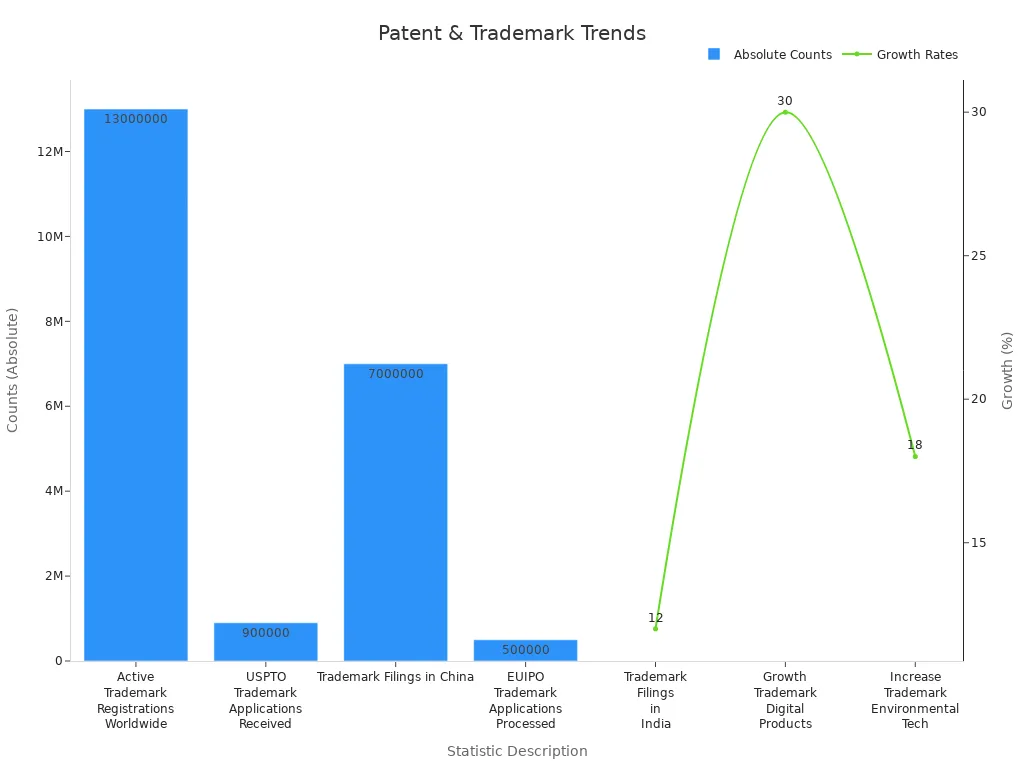
When working with Chinese manufacturers, understanding local intellectual property laws is essential. China has made significant strides in strengthening its IP framework, but I always recommend registering patents and trademarks there as well. This dual registration ensures protection in both domestic and international markets. Collaborating with local legal experts can simplify the process and help navigate China’s unique regulatory landscape.
The growing number of trademark filings in China highlights the importance of this step. In 2022 alone, over 7 million trademarks were filed, reflecting the increasing emphasis on intellectual property protection in the region.
Drafting and Using Non-Disclosure Agreements (NDAs)
Key elements of effective NDAs for manufacturers
Non-Disclosure Agreements (NDAs) are indispensable when sharing sensitive information with manufacturers. I ensure that every NDA includes key elements such as the scope of confidentiality, duration, and penalties for breaches. These agreements protect trade secrets, innovative designs, and proprietary processes, which are vital for maintaining a competitive advantage.
NDAs also enhance trust between parties. By clearly outlining confidentiality obligations, they create a secure environment for collaboration. This is especially important in exclusive orthodontic product development, where innovation drives success.
Ensuring confidentiality during design and production
Maintaining confidentiality throughout the design and production phases is crucial. NDAs safeguard technological advancements, allowing me to bring innovations to market without fear of imitation. They also mitigate risks in partnerships by establishing clear boundaries for information sharing.
For startups, NDAs play a pivotal role in attracting investors. Demonstrating a commitment to protecting intellectual property reassures stakeholders about the security of valuable assets. This proactive approach not only safeguards innovation but also strengthens business relationships.
Finding and Vetting Reliable Chinese Manufacturers
Attending trade shows and industry expos
Trade shows and expos provide another excellent avenue for finding manufacturers. Events like the International Dental Show (IDS) allow me to meet suppliers face-to-face and evaluate their offerings in real-time. These interactions help build trust and establish a foundation for long-term partnerships. I also use these opportunities to compare multiple manufacturers under one roof, saving time and effort.
At these events, I often discover innovative solutions and gain insights into emerging trends in orthodontics. For example, I recently attended IDS 2025 in Cologne, Germany, where I connected with several manufacturers showcasing cutting-edge orthodontic products. Such experiences reinforce the importance of attending industry events to stay ahead in exclusive orthodontic product development.
Evaluating Manufacturer Capabilities
Checking certifications and production capacity
Before finalizing a manufacturer, I always verify their certifications and production capacity. Certifications like ISO 13485 indicate compliance with medical device manufacturing standards, which is crucial for orthodontic products. I also assess production metrics to ensure the manufacturer can meet my requirements. Key performance indicators include:
- Yield, which measures process effectiveness.
- Manufacturing cycle time, indicating the time taken from order to finished goods.
- Changeover time, reflecting the flexibility of production lines.
These metrics provide a clear picture of the manufacturer’s reliability and efficiency. For instance, a high first-pass yield (FPY) demonstrates their ability to produce quality products consistently.
Visiting factories for on-site assessments
Whenever possible, I visit factories to conduct on-site assessments. This step allows me to evaluate the manufacturer’s facilities, equipment, and workforce. During these visits, I focus on measurable criteria such as:
| Metric | Description |
|---|---|
| Mean Time Between Failure (MTBF) | Reflects the reliability of production assets by measuring the average time between equipment failures. |
| Overall Equipment Effectiveness (OEE) | Indicates productivity and efficiency, combining availability, performance, and quality. |
| On-Time Delivery to Commit | Tracks how often the manufacturer meets delivery commitments, showcasing their operational efficiency. |
These evaluations help me identify manufacturers capable of delivering high-quality orthodontic products on time. By combining data-driven insights with personal observations, I make informed decisions that align with my business goals.
Ensuring Quality and Compliance in Manufacturing

Establishing Quality Control Processes
Setting clear quality standards and tolerances
In my experience, setting clear quality standards and tolerances is the cornerstone of manufacturing success. For exclusive orthodontic product development, I define precise benchmarks to ensure consistency and reliability. These standards guide every stage of production, from material selection to final assembly. For instance, I often use metrics like Six Sigma’s defect rate of 3.4 defects per million opportunities or the Acceptable Quality Level (AQL) to establish permissible defect thresholds. These benchmarks help maintain high-quality output while minimizing errors.
Robust quality control processes also drive operational efficiency. Tools like digital calipers and automated inspection systems enable early defect detection, ensuring that products meet stringent orthodontic standards. This approach not only reduces costs associated with rework but also enhances customer satisfaction by delivering defect-free items.
Conducting regular inspections during production
Regular inspections are essential for maintaining quality throughout the production cycle. I implement systematic checks at critical stages to identify and address issues promptly. For example, I rely on Statistical Process Control (SPC) tools to monitor trends and optimize processes. This proactive approach ensures that defects are caught early, preventing costly delays or recalls.
Inspections also provide valuable data for continuous improvement. Metrics like first-pass yield (FPY) and overall yield rates reveal process effectiveness, helping me refine production methods. By prioritizing regular inspections, I ensure that every product meets the highest standards of quality and functionality.
Meeting Industry Standards
Understanding orthodontic product regulations in target markets
Compliance with industry regulations is non-negotiable in orthodontic manufacturing. I always begin by researching the specific requirements of my target markets. For instance, the United States mandates FDA approval for medical devices, while the European Union requires CE marking. Understanding these regulations helps me design products that meet all necessary criteria, ensuring smooth market entry.
Staying informed about regulatory updates is equally important. I subscribe to industry publications and collaborate with legal experts to stay ahead of changes. This vigilance ensures that my products remain compliant, safeguarding both my business and my customers.
Working with third-party testing agencies
Third-party testing agencies play a crucial role in verifying compliance and quality. I partner with accredited organizations to conduct rigorous evaluations of my products. These agencies assess factors like biocompatibility, durability, and safety, providing unbiased validation of my manufacturing processes.
Collaborating with third-party testers also enhances credibility. Certifications from reputable agencies reassure customers and regulatory bodies about the quality of my products. This step is particularly important in exclusive orthodontic product development, where trust and reliability are paramount.
Managing Production, Logistics, and Communication
Negotiating Terms with Manufacturers
Setting pricing, MOQs, and lead times
Negotiating terms with manufacturers requires a strategic approach to ensure cost-effectiveness and smooth production. I always start by benchmarking supplier quotes to understand market pricing trends. Comparing multiple offers helps me identify competitive rates and leverage during discussions. For minimum order quantities (MOQs), I calculate them based on fixed costs divided by the contribution margin per unit. This ensures production costs are covered without overstocking, which can lead to increased holding costs.
Flexible payment terms, such as partial upfront payments, often strengthen relationships with manufacturers. These terms ease cash flow concerns for suppliers while securing favorable pricing and lead times. By balancing these factors, I achieve optimal agreements that align with my business goals.
Including penalties for delays or quality issues in contracts
Contracts must include clear penalties for delays or quality issues. I outline specific consequences, such as financial deductions or expedited rework, to hold manufacturers accountable. This approach minimizes risks and ensures timely delivery of high-quality products. For example, I recently negotiated a contract where the manufacturer agreed to a 5% discount for every week of delay. This clause incentivized punctuality and maintained production schedules.
Effective Communication During Production
Using project management tools to track progress
Effective communication is essential during production. I rely on project management tools like Trello or Asana to monitor progress and address issues promptly. These tools provide real-time updates, ensuring transparency and collaboration. Metrics such as stakeholder engagement scores and communication response times help me evaluate the effectiveness of these tools. For instance, a quick response time fosters trust and satisfaction among all parties involved.
Overcoming language and cultural barriers
Working with Chinese manufacturers often involves navigating language and cultural differences. I address this by hiring bilingual staff or using professional translation services. Additionally, I invest time in understanding cultural norms to build stronger relationships. For example, I learned that face-to-face meetings and formal greetings are highly valued in Chinese business culture. These efforts enhance mutual respect and streamline communication.
Navigating Shipping and Customs
Choosing the right shipping method for orthodontic products
Selecting the right shipping method is crucial for exclusive orthodontic product development. I evaluate options based on cost, speed, and reliability. For high-value or time-sensitive shipments, I prefer air freight due to its efficiency. For bulk orders, sea freight offers cost savings. Balancing these factors ensures timely and secure delivery.
Understanding customs regulations and import duties
Navigating customs regulations requires meticulous planning. I ensure compliance by maintaining a customs compliance rate above 95%, which avoids penalties and delays. Collaborating with customs brokers simplifies the process, as they provide expertise in documentation and import duties. For instance, understanding clearance time efficiency helps me anticipate processing durations, ensuring smooth transitions through customs.
Developing exclusive orthodontic products with Chinese manufacturers requires a structured approach. I always emphasize the importance of preparation, from defining product specifications to researching market needs. Protecting intellectual property and establishing quality control processes are equally critical. These steps ensure that every product meets industry standards and customer expectations.
To recap, here’s a summary of the key phases and methodologies involved:
| Key Phase | Description |
|---|---|
| Data Procurement | Gathering market data from various sources, including purchased databases and industry insights. |
| Primary Research | Engaging with industry experts through interviews and surveys to gather firsthand market insights. |
| Secondary Research | Analyzing published data from reputable sources to understand market trends and company performance. |
| Methodology Type | Description |
|---|---|
| Exploratory Data Mining | Collecting and filtering raw data to ensure only relevant information is retained for analysis. |
| Data Collection Matrix | Organizing data from various sources to create a comprehensive view of market dynamics. |
Taking the first step is often the hardest. I encourage you to start by researching reliable manufacturers or consulting experts in the field. With the right strategy, exclusive orthodontic product development can lead to innovative solutions and long-term success.
FAQ
What are the key benefits of working with Chinese manufacturers for orthodontic products?
Chinese manufacturers offer advanced production facilities, skilled labor, and competitive pricing. Their expertise in orthodontic product manufacturing ensures high-quality output. Additionally, their ability to scale production quickly makes them ideal partners for businesses seeking efficiency and innovation.
How can I protect my intellectual property when collaborating with Chinese manufacturers?
I recommend registering patents and trademarks in both your home country and China. Drafting comprehensive NDAs with clear confidentiality clauses is also essential. These steps safeguard your designs and innovations throughout the development process.
What should I look for when evaluating a Chinese manufacturer?
Focus on certifications like ISO 13485, production capacity, and quality control processes. Visiting factories for on-site assessments provides valuable insights into their capabilities. Metrics like on-time delivery rates and equipment reliability help determine their operational efficiency.
How do I ensure compliance with orthodontic product regulations?
Research the specific requirements of your target markets, such as FDA approval or CE marking. Partnering with third-party testing agencies ensures your products meet industry standards. Staying informed about regulatory updates helps maintain compliance over time.
What tools can I use to manage communication with Chinese manufacturers?
Project management tools like Trello or Asana streamline communication and track production progress. Hiring bilingual staff or using professional translation services helps overcome language barriers. Building strong relationships through cultural understanding enhances collaboration.
Post time: Mar-21-2025


